About us
About Urteste
Urteste specialises in the development of an innovative technology allowing the detection of cancers at early stages of their development. The Urteste motto is: Early cancer detection saves lives. The company’s ground-breaking technology helps detect cancers by measuring the activity of the enzymes in urine. Urteste develops diagnostic tests for more than ten of the most common cancers. The company’s team consists of managers with wide experience in leading medical companies and scientists who specialise in the area of proteolytic enzymes and peptide chemistry.
Vision
Our vision is to create a technology that detects many cancers at early stage of their development. Early cancer detection increases the chances of effective treatment.
Project description
Cancer can develop over a period of more than ten years without showing any warning signs. Global screening tests are currently only available for several cancer types. These mainly involve imaging tests (RTG, USG). The majority of patients are thus diagnosed too late.

Late diagnosis has a very negative effect on the development of the cancer and reduces the chances of effective treatment. For example, only 1% of patients with pancreatic cancer live for 5 years following diagnosis. When diagnosed early, pancreatic cancer can be treated surgically.
Urteste is developing ground-breaking technology for detecting cancers by measuring the activity of the enzymes in urine. The purpose of the technology is to detect multiple cancers at an early stage, using a single urine sample. The company is currently developing tests intended to detect more than ten of the most common cancers: (pancreatic, prostate, kidney, colorectal, lung, liver, endometrial, ovary, bile duct, stomach, breast, brain, esophageal, non-Hodgkin lymphoma, lukemia).
Advantages of Urteste’s technology
- Previous tests indicated high parameters in terms of test sensitivity and specificity.
- One urine sample can help detect many types of cancers.
- The test is non-invasive, safe and convenient for patients.
- The test result is available in approximately 2 hours.
- Interpretation of the result is simple and unambiguous.
- The test could be performed manually by laboratory staff or automatically using a Diagnostic Analyzer.
High demand for cancer diagnostic tests – cancers are the second leading cause of death globally.
Team
Management Board

Grzegorz Stefański
President of the Management Board
Medical doctor, manager, innovator. He has 30 years of experience in managing pharmaceutical and biotechnological companies. A co-founder of Mabion and a long-term member of the Supervisory Board. A long-term president of IBSS Kraków, where he developed and implemented innovative medical solutions for the market, including original drugs. The author of eight patents, including international ones. A graduate of the Medical University of Gdańsk and MBA studies – University of Gdańsk/Copenhagen Business School/FHTW Berlin.

Tomasz Kostuch
Member of the Board
A manager with 30 years of experience. He was a manager at several insurance companies: Warta and Ergo Hestia. He participated in the implementation of innovative projects and modern technologies. For nearly 10 years he has been involved in strategic consulting and creating financial policies for companies that carry our research and development activities. A graduate of Gdańsk University of Technology (Faculty of Electronics) and the University of Gdańsk (Faculty of Management).
Scientific Board

Prof. Adam Lesner
President of the Scientific Board
The originator of the Urteste project. He has 30 years of experience in chemical sciences. A globally-recognised figure in research on proteolytic enzymes and peptide chemistry. For the past few years, he has been focusing on creating diagnostic tests that allow the early diagnosis of various conditions, including cancer, through the analysis of specific proteolytic activity. The author of over 150 original and review scientific papers published in journals included on the “Philadelphia list”. He is an editor of PLOS One and Open Biochemistry Journal. He is the author of four international and national patents and four patent applications. His works have been quoted over 1,000 times.

Natalia Gruba PhD
A chemist with 15 years of experience. She has been successfully developing diagnostic methods using protease activity. The results of her studies were published in scientific papers included on the “Philadelphia list”. She is the author of three patents, including an international one and two patent applications.
Research and Development Center
Urteste operates its own research and development center where technology for early cancer diagnostics is developed. Our Center consists of:
- Biobank
- Chemical Laboratory
- Diagnostic Laboratory
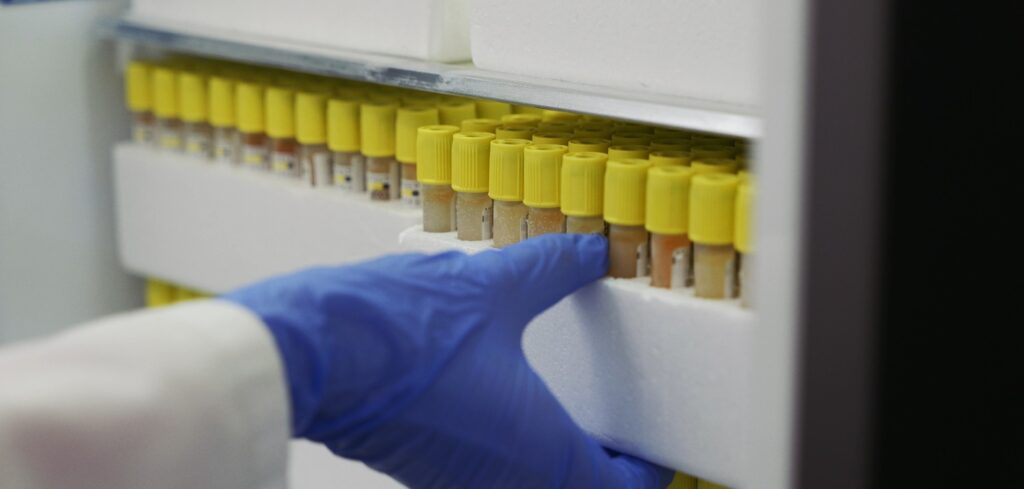
Biobank
The Biobank is an essential element of our R&D Center and serves as a carefully curated source of diverse biological samples. These resources, along with our patented compounds, form the foundation of innovative research projects developed by Urteste.
Chemical Laboratory
At our Research and Development Center, we specialize in the chemical synthesis of reagents and buffers crucial for development of our cancer detection tests.
Equipped with the latest peptide synthesizer apparatus and employing advanced analytical methods, our laboratory ensures the production of final products with exceptional purity levels. Trust us to deliver innovative solutions for cancer detection.

Diagnostic Laboratory
Our laboratory ensures the accuracy and reliability of our tests by carefully checking their analytical parameters. Equipped with cutting-edge technology, including advanced automated pipetting solutions and integrated absorbance readers, to make sure our tests are exact and quick providing test results in approx. 2 hours.


